Know The Stereoisomers in Organic Chemistry: A Broad Overview Of Stereochemistry
Organic chemistry is so varied, especially when considering the concept of stereoisomers. It's rather fascinating to see how molecules can have variations based on spatial orientations of the atomic arrangement. Stereoisomers, after all, are not just about how atoms are connected, but also about the orientation of those connections in three-dimensional space.
Spatial Arrangement of Organic Molecules
Spatial arrangement determines the properties of organic molecules, in much the same way that a small variation in design can markedly alter the function of a product. The way atoms are arranged will influence how a given molecule interacts with its surroundings, making an understanding of stereoisomers essential to chemistry and a host of other disciplines.
Stereoisomers: Definition More than Structural Isomers
Steroisomers are the chemical compounds that share the same formula and even connectivity of the atoms but have a different arrangement in space. Such a difference leads to differences in some physical and chemical properties of the compounds. There are two major categories of stereoisomers: enantiomers and diastereomers.
A Glimpse into Applications and Importance of Stereoisomerism
Stereoisomerism has wide implications:
It is of utmost important during drug design, one stereoisomer may be active and useful, while the other being toxic or noneffective .
Stereoisomers are very important to flavor chemistry, as they determine the way we perceive taste and smell.
Enantiomers: Non-Superimposable Mirror Images
Defining Chirality and the Presence of Chiral Centers
Enantiomers are a special and unique type of stereoisomer that are the non-superimposable (molecule doesn't show any symmetry) mirror images of each other. This results from a chiral centre typically of a carbon atom bonded to four different groups.
Chirality: An Essential Property in Chemistry
Chirality, an abstract concept in chemistry, actually happens to be the very basis for understanding the properties and behavior of molecules. Essentially, chirality is the property of asymmetry in molecules, specifically when the mirror image of a molecule is not superimposable on itself. This is caused by four different kinds of substituents being attached to a central atom or group of atoms resulting in the formation of two non-identical mirror images known as enantiomers.
History of Chirality
The term "chirality" (which comes from The Greek) refers to the way things a molecule (like a human hand) can be different from their mirror images. Just as our two hands cannot be overlapped one over the other despite the similarity between them in their structure, chiral molecules exhibit chirality. This property, arising from the spatial arrangement of atoms within the molecule, often has considerable consequences in applications from pharmacology and biology to materials science.
Enantiomers: Mirror Images with Different Properties
Enantiomers are pairs of molecules that have the same chemical structure but differ in three dimensional orientation. Such similar molecules can have widely different effects on biological systems. Classic examples are thalidomide-in one of its enantiomers, administered to pregnant women, it helped control morning sickness; while its mirror image caused severe birth defects.
This is because enzymes and receptors in living organisms prefer to react with one enantiomer more than its counterpart because of the difference in spatial arrangement. Thus, knowledge of chirality is essential for drug design so that pharmaceuticals may be safer and effective.
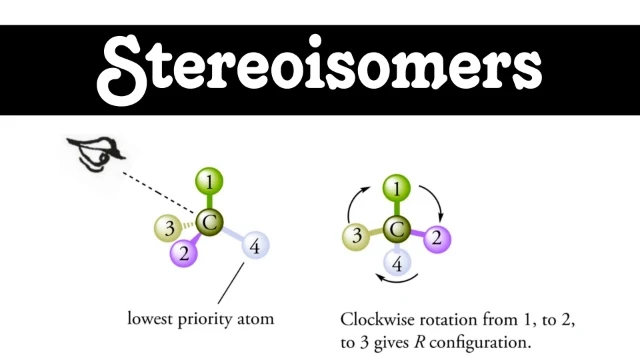
Determining Chirality: Cahn-Ingold-Prelog Rules
The chemists use the Cahn-Ingold-Prelog (CIP) rules to determine from the aspect of stereochemistry whether a molecule is chiral or achiral. According to them:
Prioritize by atomic number:
Align that molecule so that the lowest-priority substituent or group is positioned away from you. However, this step is crucial, because it allows for accurate spatial orientation. Although it may seem simple, proper alignment is essential for understanding the molecular structure.
Draw a path from highest priority to lowest. If it looks clockwise, it is R configuration and is called R configuration; counterclockwise gives S configuration.
These rules should be applied with a structured approach to each chiral center within a molecule in order to predict its overall chirality and how it is likely to react with other compounds.
Nature Importance of Chirality
Chirality is an important concept in nature, both macroscopically and at the microscopic level. Biology at the molecular level shows many vital molecules as chiral in structure-amino acids and sugars are important examples: All amino acids present in proteins exist only as the L-enantiomers while sugars assume most frequently the D-configurations.
In reality, DNA itself is chiral, arising from its right-handed helical structure – further evidence that nature favors certain orientations of molecules, which are determined by chirality rules.
Going to larger scales, phenomena like polarized light will interact in different manners with chiral compounds and may show striking implications for even tiny differences between enantiomers in physical interactions in natural processes.
Synthesis Challenges by Chiral Molecules
Synthesis of chiral compounds has been a significant challenge for organic chemists because of the problems associated with control of the stereoselectivity during reactions involving asymmetric carbon centers or other chiral elements within molecules.
Traditional approaches rely on separation of racemic mixtures into individual enantiomers after synthesis or employing chiral catalysts/auxiliaries in reactions; the methods are often associated with low yields or expensive separation techniques to acquire the desired chiral products.
Some of the more modern developments, for example organocatalysis and biocatalysis offer far greener routes to obtain single-enantiomer products in one step, thereby again underlining ongoing efforts by scientists in making the chemistry of chiral compounds less cumbersome.
Chirality is the concept which underlies all of scientific work-a pillar that crosses chemistry, biology, pharmacology, and materials science. Its implications reach beyond consideration of symmetry, but chiral interactions between substances in molecular levels with farther consequences duly manifest in nature and humanly developed endeavors.
It is only an understanding of chirality's subtleties that lets scientists take advantage of its potential-from safer drugs to the development of new applications in other areas of industry that follow from the precise molecular recognition processes it follows.
And further innovation-driven solutions solving synthetic challenges posed by chiral compounds,
we open new avenues toward better therapeutics, novel materials, and deeper understanding of the complex biochemical processes governed by chirality.
Determining Enantiomers Using the R/S Configuration System
Enantiomers (which are molecules that are mirror images of each other) can be identified (with precision) using the R/S configuration system: this method is essential for understanding their properties. A chiral centre can be split and classified according to the priority of attached groups. The configurations can be either:
R (Rectus): Right-handed
S (Sinister): Left-handed
Examples of Enantiomers in Pharmaceuticals: Thalidomide
One great example is thalidomide, where one enantiomer was effective for morning sickness but the other caused horrible birth defects. Such a case emphasizes how stereochemistry is critical in designing drugs.
Diastereomers: Stereoisomers that are Not Mirror Images
Defining Diastereomers and their Relationships
Unlike the enantiomers, the diastereomers are stereoisomers that do not have a mirror relationship to each other. They can differ significantly in melting point and solubility (among other physical properties).
Geometric Isomerism (cis-trans) as a Type of Diastereomerism
Geometric isomerism is also referred to as a cis-trans isomerism that describes how atoms are arranging in space in a molecule. It is a diastereomer type. For instance:
Cis isomers: when same type of Atoms or groups are situated on the same side of the double bond chain or ring.
Trans isomers: same Atoms or groups are positioned on opposite sides of the double bonded chain or ring.
Diastereomers in an example that can be observed in fatty acids, where changes in the geometric structure greatly affect physical properties and biological activity.
Optical Activity and Polarimetry
Plane-Polarized Light and Optical Rotation
Stereoisomers react differently to plane-polarized light. Enantiomers can rotate the plane of light (The plane of polarization) in opposite directions. The extent to which they do is termed optical rotation.
Measuring Optical Rotation Using a Polarimeter
Scientists use a tool called a polarimeter to measure optical rotation. This device determines how much light rotates when passing through certain substances.Chemists make use of this method to separate different enantiomers as dictated by the angle by which light is rotated by the sample under test.
Importance of Specific Rotation in Describing Enantiomers
It has the specific rotation, therefore a characteristic property of a compound and very useful in its identification. The observed-opposite rotation is divided by the concentration of the solution and the path-length of light used in polarimeter.
Meso Compounds: A Special Case of Stereoisomers
Defining Meso Compounds and their Internal Symmetry
Meso compounds are very unique because they have more than one chiral center but are still classifies achiral because there is a plane of symmetry and we call them meso compounds. They are not optically active.
Identifying Meso Compounds: Achiral Despite Having Chiral Centers
To determine meso compounds, look for an internal plane of symmetry in the given compound if there are 2 chiral centers & RS=SR then it will be meso compound. Even though a molecule has chiral centers, it is considered meso if it otherwise has symmetry.
Examples of Meso Compounds and their Properties
Meso tartaric acid is another typical example; its overall symmetry makes it achiral despite the two chiral centres it contains, thus demonstrating how structure impacts properties.
Applications and Significance of Stereoisomerism
Stereoisomerism in Drug Discovery and Design
Pharmaceutical industry seeks the right stereoisomer which may increase the therapeutic effect and decrease the side effects.
Stereoisomers in Food Science and Flavor Chemistry
Stereoisomers play an important role in food science and flavor chemistry.
In food science, the flavour and odor of certain substances are determined by stereoisomerism. Some isomers have pleasant odors or flavors.
Stereoisomer Future Work: Statistics on Current Research
There are ongoing works into stereoisomers' future research potential. With advancing technologies, it is possible to synthesize and determine these compounds.
Conclusion: Main Points to Know More About and Further Investigation
In summary, enantiomers are non-superimposable mirror images, whereas diastereomers aren't Non-superimposable. Any student of organic chemistry would need to know this information.
Stereoisomerism is prevalent in medicine to food and anything in between. The knowledge of these can help make one understand organic compounds at a more profound level.
Resources to Read and Learn More Steroisomers
For those interested in delving deeper into the study of stereoisomers, one may want to look at texts on organic chemistry, online courses or even scientific journals about stereochemistry.
















0 Comments