A Comprehensive Guide to Carbonyl Compounds and Their Condensation Reactions
Introduction
Carbonyl compounds are important molecules in organic chemistry. They have a unique structure with a carbon-oxygen double bond (C=O) and can undergo condensation reactions to form more complex structures.
These reactions are crucial for various chemical processes, both natural and synthetic, including the production of drugs and fragrances.
This guide will cover:
- The basic structure and properties of carbonyl compounds
- Different types of condensation reactions and how they work
- Real-life applications in organic synthesis
- Their importance in biology and future research possibilities
Whether you're studying chemistry, conducting research, or simply interested in the subject, understanding these reactions can lead to:
- The ability to create intricate molecules from simpler ones
- The discovery of new ways to synthesize compounds
- Insights into biological mechanisms
- Advancements in drug development
Join us as we delve into the world of carbonyl compounds and their condensation reactions, uncovering their significance in organic chemistry.
Understanding Carbonyl Compounds
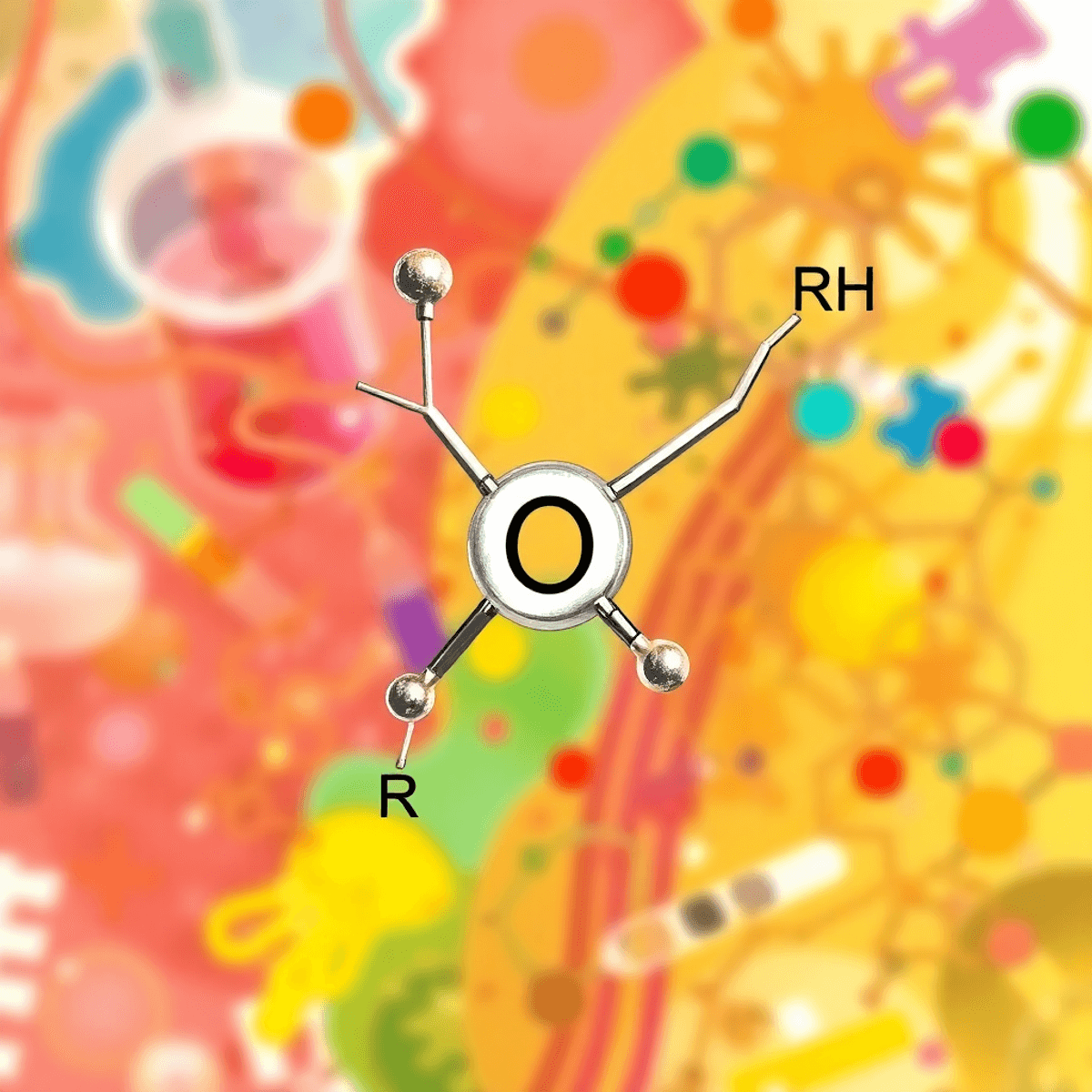
The carbonyl group is one of the most interesting functional groups in organic chemistry. It consists of a carbon atom double-bonded to an oxygen atom (C=O), which creates a reactive center that can undergo various chemical reactions.
Structure of Carbonyl Compounds
The structure of carbonyl compounds can be visualized as:
R1-CO-R2
Where R₁ and R₂ represent different groups attached to the carbonyl carbon.
Types of Carbonyl Compounds
Based on the nature of the R groups, carbonyl compounds can be classified into two main categories: aldehydes and ketones.
Aldehydes
Aldehydes have at least one hydrogen atom directly attached to the carbonyl carbon. Some common examples include:
- Formaldehyde (H-CHO) - used in preservatives
- Acetaldehyde (CH₃-CHO) - present in coffee aroma
- Benzaldehyde (C₆H₅-CHO) - gives almond its characteristic smell
Ketones
Ketones have two carbon-containing groups attached to the carbonyl carbon. Some common examples include:
- Acetone (CH₃-CO-CH₃) - nail polish remover
- Benzophenone (C₆H₅-CO-C₆H₅) - UV absorber in sunscreens
- Cyclohexanone - precursor in nylon production
Reactivity of Carbonyl Compounds
The unique electronic structure of the carbonyl group creates a polar bond between carbon and oxygen. This polarity makes the carbon atom electrophilic (electron-deficient) and the oxygen atom nucleophilic (electron-rich). As a result, carbonyl compounds can participate in various chemical reactions, including:
- Nucleophilic addition reactions
- Enol and enolate formation
- Condensation reactions
- Reduction to alcohols
- Reaction with Grignard reagents
Importance of Carbonyl Compounds
Carbonyl compounds are not only important in the laboratory but also have significant roles in various fields such as:
- Biological processes (proteins, carbohydrates)
- Industrial manufacturing (polymers, pharmaceuticals)
- Food chemistry (flavors, aromas)
- Natural product synthesis
Types of Condensation Reactions Involving Carbonyl Compounds
Carbonyl compounds participate in diverse condensation reactions, creating complex molecular structures through carbon-carbon bond formation. These reactions serve as fundamental tools in organic synthesis.
Aldol Reactions
The aldol reaction represents a powerful synthetic method where two carbonyl compounds join forces to create β-hydroxy aldehydes or ketones. This reaction gets its name from the combination of "aldehyde" and "alcohol," reflecting the key functional groups involved.
Basic Mechanism of Aldol Reaction:
- Enolate FormationBase removes α-hydrogen
- Creation of resonance-stabilized enolate ion
- Enhanced nucleophilicity at α-carbon
- Nucleophilic AdditionEnolate attacks electrophilic carbonyl carbon
- Formation of carbon-carbon bond
- Generation of alkoxide intermediate
- ProtonationAlkoxide accepts proton
- Formation of β-hydroxy carbonyl product
- Stabilization through hydrogen bonding
The reaction can proceed further through dehydration:
Under acidic or basic conditions, β-hydroxy aldehydes lose water to form α,β-unsaturated carbonyl compounds, introducing valuable conjugated systems into molecules.
Types of Aldol Reactions:
- Intramolecular Aldol Reactions: Single molecule contains both carbonyl groups, forms cyclic products (Example: Formation of cyclopentanone derivatives)
- Mixed Aldol Reactions: Two different carbonyl compounds react, requires careful control of conditions (Example: Reaction between acetaldehyde and propanone)
Practical Considerations:
- Temperature control affects product distribution
- Base strength influences reaction rate
- Solvent choice impacts selectivity
- Steric hindrance determines reactivity patterns
Real-World Applications:
- Pharmaceutical synthesis
- Natural product construction
- Industrial chemical production
- Fragrance compound creation
The aldol reaction's versatility stems from its ability to create new carbon-carbon bonds while introducing functional groups that serve as handles for further transformations.
Claisen Condensation
The Claisen condensation is a powerful synthetic tool in organic chemistry. It allows chemists to create new carbon-carbon bonds by reacting two ester molecules or an ester with other carbonyl compounds. This reaction produces β-keto esters, which are valuable building blocks for synthesizing complex organic molecules.
Key Features of Claisen Condensation:
- Forms β-keto esters as primary products
- Requires strong base catalysts (like sodium ethoxide)
- Involves ester substrates as key reactants
- Creates new carbon-carbon bonds
The mechanism of Claisen condensation is similar to the aldol reaction but has some distinct characteristics:
- Base-Induced Enolate Formation: A strong base removes the α-hydrogen from the ester, creating a nucleophilic enolate intermediate.
- Nucleophilic Addition: The enolate attacks the carbonyl carbon of the second ester, forming a tetrahedral intermediate.
- Elimination Step: The alkoxide group leaves, producing the β-keto ester product.
Practical Applications:
- Synthesis of 1,3-dicarbonyl compounds
- Production of pharmaceutical intermediates
- Creation of flavor and fragrance compounds
The versatility of this reaction goes beyond simple esters. Mixed Claisen condensations can occur between different ester molecules, leading to diverse product possibilities. To achieve optimal yields, it is crucial to control the temperature and carefully select the base catalyst.
Synthetic Advantages:
- High yields under controlled conditions
- Predictable reaction pathways
- Minimal side product formation
- Scalable to industrial processes
Recent developments in Claisen condensation methodology include:
- Green chemistry approaches using ionic liquids
- Asymmetric variations for stereoselective synthesis
- Microwave-assisted protocols for faster reactions
- Novel catalyst systems for improved selectivity
The importance of this reaction in organic synthesis continues to grow as new applications emerge in drug discovery and materials science.
Understanding the Mechanism of Carbonyl Condensation Reactions
Carbonyl condensation reactions are complex processes involving the movement of electrons. These reactions are controlled by specific mechanisms that determine how they occur. One key player in these reactions is the enolate ion, which acts as a strong nucleophile and drives the reaction forward.
How Enolate Ions Form
Enolate ions are formed through a delicate balance between acids and bases:
- The hydrogen atom on the α-carbon becomes more acidic because of the nearby carbonyl group.
- A base catalyst removes this hydrogen atom, resulting in a negatively charged ion stabilized by resonance.
- The enolate ion that is formed can exist in two different forms: O-form and C-form.
The Impact of Enolate Stability on Reactions
The stability of enolate ions has a direct influence on how quickly reactions occur and what products are formed:
- More substituted enolates are generally more stable.
- Enolates with extended conjugation (alternating double bonds) also show increased stability.
- The presence of neighboring groups can have electronic effects that affect the formation of enolates.
Reversibility of Carbonyl Condensation Reactions
It's important to note that carbonyl condensation reactions are inherently reversible. This means that under certain conditions, the reaction can go both ways - from reactants to products and vice versa. Several key factors influence this equilibrium position:
Temperature Control
- Higher temperatures tend to favor the reverse reaction (products converting back into reactants).
- Lower temperatures help stabilize the products.
Concentration Effects
- Removing water or any other products from the reaction mixture will drive the reaction forward towards forming more products.
- If there is an excess amount of reactants present, it will shift the equilibrium towards producing more products.
pH Considerations
- Maintaining an optimal pH range is crucial for keeping the catalyst active.
- If the conditions become too basic (high pH), there is a risk of unwanted side reactions occurring.
- On the other hand, if the conditions become too acidic (low pH), it can suppress the formation of enolates.
The Importance of Catalytic Conditions
The success of carbonyl condensation reactions heavily relies on the specific catalytic conditions used:
Small amounts of base catalyst generate sufficient enolate concentration without promoting unwanted side reactions.
By understanding these mechanistic details, chemists gain valuable insights into how they can control these reactions effectively. This knowledge allows them to:
- Selectively produce desired products
- Maximize yields (amounts produced)
- Minimize unwanted byproducts
- Develop new synthetic strategies (methods for making compounds)
The interaction between thermodynamics (energy changes) and kinetics (reaction rates) creates unique profiles for these reactions. Through careful manipulation of various reaction conditions such as temperature, concentration, and pH, chemists can achieve precise control over their outcomes both in laboratory experiments and industrial processes.
Applications of Carbonyl Compound Condensation Reactions
The versatility of carbonyl compound condensation reactions has transformed both synthetic chemistry and our understanding of biological processes. These reactions are powerful tools for creating complex molecular structures and supporting essential functions in living organisms.
Applications in Synthetic Chemistry
1. Drug Development
- Creation of β-lactam antibiotics through aldol condensations
- Synthesis of anti-inflammatory compounds
- Production of cholesterol-lowering medications
2. Natural Product Synthesis
- Building complex terpenes and alkaloids
- Construction of macrocyclic compounds
- Formation of polyketide frameworks
3. Materials Science
- Development of biodegradable polymers
- Creation of novel optical materials
- Synthesis of conducting organic materials
Role in Biological Systems and Metabolic Pathways
The significance of carbonyl condensations extends deep into cellular metabolism:
1. Citric Acid Cycle
- Aldol-type reactions catalyzed by aldolase enzymes
- Formation of crucial metabolic intermediates
- Energy production regulation
2. Fatty Acid Synthesis
- Claisen condensation reactions in chain elongation
- Production of essential lipid molecules
- Membrane component synthesis
3. Secondary Metabolism
- Biosynthesis of flavonoids, a process that has been extensively studied for its implications in plant defense mechanisms
- Production of defensive compounds in plants
- Generation of signaling molecules
Industrial Applications
Carbonyl compound condensation reactions find applications in various industries:
1. Food Industry
- Flavor compound synthesis
- Production of food preservatives
- Creation of artificial sweeteners
2. Fragrance Manufacturing
- Synthesis of aromatic compounds
- Production of perfume ingredients
- Development of new scent molecules
These reactions continue to expand the possibilities in chemical synthesis, allowing for the creation of increasingly complex molecules while imitating nature's elegant synthetic pathways. Research teams worldwide utilize these transformations to develop new drugs, materials, and industrial processes, highlighting the lasting importance of carbonyl condensation chemistry.
In addition to these applications, carbonyl compound condensation reactions also play a significant role in organic chemistry education, providing students with practical examples and case studies that enhance their understanding and appreciation for this vital area of study. Furthermore, the wealth of knowledge generated from these reactions continues to be shared at forums such as the ACS National Meetings, fostering collaboration and innovation in the field.
Future Directions in Research on Carbonyl Compound Reactions
The field of carbonyl compound research is constantly evolving, creating new opportunities for scientific exploration. Here are some promising areas that are currently being studied:
1. Green Chemistry Applications
- Development of water-based reaction systems
- Creation of biodegradable catalysts
- Implementation of solvent-free condensation methods
2. Asymmetric Synthesis
- Design of novel chiral catalysts
- Exploration of stereoselective condensation reactions
- Enhancement of enantioselectivity in carbonyl transformations
3. Computational Chemistry
- Modeling of reaction mechanisms using AI
- Prediction of optimal reaction conditions
- Virtual screening of potential catalysts
4. Biological Applications
- Creation of biomimetic catalysts
- Development of enzyme-inspired synthetic methods
- Integration with protein engineering
The combination of carbonyl chemistry and emerging technologies offers exciting possibilities. Scientists are looking into photocatalytic methods for carbonyl condensations, while others are focusing on flow chemistry techniques for large-scale industrial applications. Additionally, controlled carbonyl chemistry is being explored as a way to develop smart materials.
There is also great potential in researching new catalyst designs. Scientists are investigating metal-organic frameworks, supported catalysts, and recyclable systems to improve reaction efficiency and sustainability. These advancements have the potential to transform pharmaceutical synthesis and materials science, expanding the limits of what can be achieved in organic chemistry.
FAQs (Frequently Asked Questions)
What are carbonyl compounds and why are they significant in organic chemistry?
Carbonyl compounds are organic molecules that contain a carbonyl group (C=O), which is a functional group consisting of a carbon atom double-bonded to an oxygen atom. They include aldehydes and ketones, which play crucial roles in various chemical reactions and synthesis processes. Understanding these compounds is essential for organic synthesis and the creation of complex molecules.
What are the main types of condensation reactions involving carbonyl compounds?
The






0 Comments